 TDI (Toluene diisocyanate) is the vital raw material of mattress foam manufacturers but its availability and market and price stability are headaches for the manufacturers.
TDI (Toluene diisocyanate) is the vital raw material of mattress foam manufacturers but its availability and market and price stability are headaches for the manufacturers.
Short supplies of this key ingredient in polyurethane foam seem not to be easing soon. There are few manufacturers in the world and when they have self-problems they are reflected to users.
As the main input of foam, Toluene Diisocyanate (TDI) is one of the basic chemical ingredients used in the manufacture of flexible polyurethane foam. TDI is the abbreviation for Toluene Di-Isocyanate, a mixture of toluene-2,4-diisocyanate and toluene-2,6-diisocyanate with the 2,4- isomer being the major component.
Polyurethane chemistry was first studied and produced in Germany in 1937. Polyurethanes represent a family of polymers rather than a single polymer. Urethane polymers can be produced with a wide variety of properties, ranging from soft flexible foams and fibres through to hard solids so that they can be used in a diverse range of applications. The TDI is consumed in the chemical reaction that makes polyurethane and is not released from the finished polyurethane foam into the air. Flexible polyurethane foam is used in mattresses, furniture cushions and other common household applications.
 The development of elastic polyurethanes began as a program to find a replacement for rubber during the days of World War II. In 1940, the first polyurethane elastomers were produced. These compounds gave millable gums that could be used as an adequate alternative to rubber. When scientists found that polyurethanes could be made into fine threads, they were combined with nylon to make more lightweight and stretchable garments.
The development of elastic polyurethanes began as a program to find a replacement for rubber during the days of World War II. In 1940, the first polyurethane elastomers were produced. These compounds gave millable gums that could be used as an adequate alternative to rubber. When scientists found that polyurethanes could be made into fine threads, they were combined with nylon to make more lightweight and stretchable garments.
Polyurethanes are linear polymers that have a molecular backbone containing carbamate groups (-NHCO2). These groups, called urethane, are produced through a chemical reaction between a diisocyanate and a polyol. Polyurethanes are some of the most versatile polymers. Despite these differing properties, the polymers have one common characteristic in that they all incorporate the urethane group (-NH-CO-O-) into their structure. However, the polymers differ from simple thermoplastic polymers, such as the polyolefins, in that they are not sold as ready-made polymers but as precursors that are mixed at the conversion stage. These precursors are commonly polyols (compounds containing multiple -OH groups) and diisocyanates (compounds containing -NCO groups). The primary reaction during the production of polyurethanes is of the form: -NCO + HO- → -NH-CO-O-
Polyurethane flexible foams can be used for a diverse range of applications including sofa, mattresses, furniture cushions, carpet underlay, padding for textile and footwear, and automotive seats. TDI is also used in the production of coatings, sealants and elastomers.
Toluene Diisocyanate TDI 80-20 is an aromatic isocyanate that is produced for reaction with polyols to form polyurethanes. It is widely used in the production of polyester-based soft foam, high-bearing sponges, semi-rigid ester foam, and paint.
What doe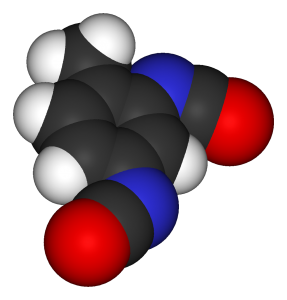 s the presence of TDI antibodies mean?
s the presence of TDI antibodies mean?
The presence of TDI antibodies does not necessarily indicate an adverse health effect, nor does it necessarily mean you have been exposed to TDI. The test for TDI antibodies is neither simple nor completely accurate. Some individuals test positive regardless of whether they have been exposed to TDI or not. Additional testing would be required to determine if the positive reading was actually the result of TDI exposure.
Environmental implications
TDI is a confirmed carcinogen. Its emission is closely monitored by the authorities. TDI tape detectors are installed around the factories it is produced. To minimize the emission of TDI, most of the process equipment is shielded.
Like many reactive chemicals, “TDI products” can create hazards if handled carelessly. When used in accordance with current regulations and industry practices, TDI can be used safely. Engineering controls, personal protective equipment, and long-established workplace practices are widely used throughout the industry to protect workers and the public.
Traditionally CFC (chlorinated fluorocarbons) based solvents were used as blowing agents. In the late 80’s, the ozone layer depletion effect of CFC based chemical was established. The use of CFC was gradually banned. Methylene chloride, which is inert to ozone, is used as a substitute for CFC.
Lots of “offcuts” are generated in the foam cutting process. They are collected and sent to the associated recycle plant to produce a lower grade flexible foam. This type of foam is widely used in the production of carpet backings and packing materials. By this way the net amount of waste produced in the process can be minimized.
TDI should only be handled by knowledgeable, well-trained personnel who thoroughly understand the hazards associated with the transportation, storage, and use of the chemical. Contaminated clothing must be washed before reuse. Severely contaminated clothing should be discarded. Contaminated footwear or leather gloves should never be reused. Eating and drinking should not be allowed where TDI is handled or stored.
 POLYOL in polymer chemistry used in mattresses
POLYOL in polymer chemistry used in mattresses
The other reacting species required to produce polyurethanes are compounds that contain multiple alcohol groups (OH), called polyols. In polymer chemistry, polyols are compounds with multiple hydroxylfunctional groups available for organic reactions. A molecule with two hydroxyl groups is a diol, one with three is a triol, one with four is a tetrol and so on.
The polyols used in polyurethane production are predominantly hydroxy- polyethers, rather than hydroxy-polyesters. They are produced by alkoxylation. Depending on the degree of cross-linking required, the starting alcohols used for hydroxy-polyethers may be divalent glycols (ethylene, propylene and other glycols) or multivalent alcohols (e.g. glycerol, sucrose). The epoxides used are generally propylene oxide and ethylene oxide.
Monomeric polyols such as glycerin, pentaerythritol, ethylene glycol and sucrose often serve as the starting point for polymeric polyols. These materials are often referred to as the “initiators” and reacted with propylene oxide or ethylene oxide to produce polymeric polyols. However, they should not be confused with free radical “initiators” used to promote other polymerization reactions. The functional group used as the starting point for a polymeric polyol need not be a hydroxyl group; there are a number of important polyols which are built up from amines. A primary amino group (-NH2) often functions as the starting point for two polymeric chains, especially in the case of polyether polyols.
Polymeric polyols are generally used to produce other polymers. They are reacted with isocyanates to make polyurethanes used to make mattresses, foam insulation for appliances (refrigerators and freezers), home and automotive seats, elastomeric shoe soles, fibers (e.g. Spandex), and adhesives.
Polymeric polyols are usually polyethers or polyesters. Polyether polyols are made by reacting epoxides like ethylene oxide or propylene oxide with the multifunctional initiator in the presence of a catalyst, often a strong base such as potassium hydroxide or a double metal cyanide catalyst such as zinc hexacyanocobaltate-t-butanol complex. Common polyether diols are polyethylene glycol, polypropylene glycol, and poly(tetramethylene ether) glycol. The examples shown below are fairly low molecular weight triols based on glycerin (a triol) being reacted with propylene oxide, ethylene oxide or a combination of the two. In reality, the chains would not be of equal length in any one molecule and there would be a distribution of molecular weight polyols within the material. Polyether polyols account for about 90% of the polymeric polyols used industrially; the balance is polyester polyols.
As is the case for all foam manufacturers at almost all over the world Turkish foam manufacturers have also been suffering some supply problems of this main input of their manufacturing process. Price hikes are also a big problem for the industry. Depending on this material for foam production the industry is almost in a monopoly of a main supplier. When it suffers from any cause the entire industry suffers because the producer naturally reflects the loss on prices, delivery and other factors.
Another class of polymeric polyols is the polyesters. Polyesters are formed by condensation or step-growth polymerization of diols and dicarboxylic acids (or their derivatives), for example diethylene glycol reacting with phthalic acid.[5] Alternatively, the hydroxyl group and the carboxylic acid (or their derivatives) may be within the same molecule, as in the case of caprolactone. The example below is an idealized structure that could be obtained by reacting pentaerythritol (a tetrol) with gamma-butyrolactone.
#manset, #TDI (Toluene dilsocyanate), #Toluene Di-Isocyanate, #POLYOL, #antibodies, #Traditionally CFC
 SleepTech Magazine Mattress, Accessories, Machinery, Raw Materials
SleepTech Magazine Mattress, Accessories, Machinery, Raw Materials




Dear sir/Madam
Greetings to you?
This is to inform you that I want to buy tdi 80 20
please get back us for more details.
Thanks
Tel: +256759510150
Message: Dear Sir/Madam
We are procurement company, we have clients interested in purchasing, TDI FORM ,etc in big quantity but our clients use 100% OA 90 /CAD days bill date. if you work above payment back to us.
Regards
Nabaweesi
MARKSAM CONSTRUCTION CO. (UG) LTD
P.O. BOX 12416, KAMPALA UGANDA EST AFRICA
email: [email protected]
FAX: +256 753 425568
TEL: +256 752 525 909
MARKSAM
Hey there! I could have sworn I’ve been to this site before but after checking through some of the post I realized it’s new to me. Anyhow, I’m definitely happy I found it and I’ll be book-marking and checking back often!
Please keep sharing : https://www.chemocart.com/search?search=Toluene&search_result=all
Also read my blog : http://blog.chemocart.com/understanding-all-about-toluene/
Hi there, I found your site via Google while searching for a related topic, your site came up, it looks great. I’ve bookmarked it in my google bookmarks.
Please keep sharing :https://www.chemocart.com/view_product/toluene/185/46